The excellent physicochemical properties exhibited by silica nanoparticles (SiNPs) have led to their wide acceptance in industrial and biomedical applications. Alongside the growing popularity of SiNPs is also the increasing concern regarding its negative effects on human health. To enable a better understanding of SiNPs’ toxicity towards biological material, researchers from Chiba University now propose a technique that can separate nanoparticles of different sizes, assess their uptake by cells, and evaluate the potential toxicity.
Metal nanomaterials have become an indispensable part of industrial and medical fields due to their unique and versatile properties. Their size, which imparts them with the desired physiochemical properties, is also the reason for environmental and health concerns. The nano-sized particles in nanomaterials have shown high reactivity towards biomolecules and often even toxicity towards biological cells.
Scientists have attributed this behavior of metal nanoparticles in interaction with biomolecules to phenomena like inflammation or oxidative stress. However, to ensure the safe usage of metal nanoparticles, there is a need to explore molecular mechanisms responsible for the toxicity and understand how the uptake of nanoparticles by cells varies based on their shape, size, morphology, and other aspects.
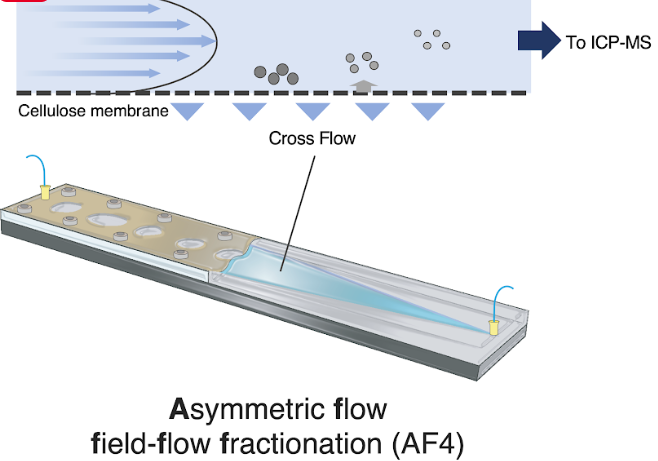
To shed light on this issue, Assistant Professor Yu-ki Tanaka and Prof. Yasumitsu Ogra, both from the Graduate School of Pharmaceutical Sciences at Chiba University, have now estimated the cellular intake of silica nanoparticles (SiNPs) based on their sizes. In their recent breakthrough published in Archives of Toxicology on January 14, 2024, the researchers developed an AF4-ICP-MS (asymmetric flow field flow fractionation with inductively coupled plasma mass spectrometry) system, which separated SiNPs of five different sizes (10, 30, 50, 70, and 100 nm) and enabled quantitative assessment of cytotoxicity of SiNPs in HepG2 cells.
“SiNPs have gained momentum in various fields such as drug delivery, biomedical imaging, catalysts as well as environmental remediation for removing contaminants from water and soil. However, there is also a significant concern about its environmental toxicity and potential impact on living organisms,” says Dr. Tanaka when asked about the motivation behind this study. “So, to find a remedy to the trade-off between industrial availability and toxicity, we decided to develop a technique to understand the potential adverse effects of SiNPs by combining quantitative data on cellular uptake and toxicological responses.”
Size analysis techniques like electron microscopy and laser-based dynamic light scattering failed to observe nanoparticle specimens in deep layers and elucidate the chemical compositions of the nanoparticles. To counter these issues, the team adopted the new AF4-ICP-MS size analysis technique, which not only overcame those issues but also detected nanoparticles of size as low as 10 nm. This wouldn’t have been possible with conventional ICP-MS methods.
The team used the AF4-based method to evaluate the cellular uptake of SiNPs in lab-cultured human hepatoma HepG2 cells. The measurements showed that approximately 17% of the SiNPs exposed to the HepG2 cells were absorbed. The transmission electron microscopy (TEM) carried out by the team observed the presence of SiNP aggregates within the cells, indicating the ability of small nanoparticles to settle down in the culture medium and enter the cells easily.
“We found that the smaller SiNPs exhibited higher toxicity towards the HepG2 cells than the larger ones, but the AF4-ICP-MS analysis found no significant size-dependent difference in the particle volume absorbed by the cells,” remarks Dr. Tanaka, highlighting the outcomes of the toxicity experiments. These results suggested that the elevated cytotoxic behavior of the small SiNPs was rooted in the large surface area relative to particle volume when compared to the larger ones.
The researchers also investigated the chemical mechanisms associated with cytotoxicity. The data indicated that cell necrosis was partly linked to oxidative stress caused by the production of reactive oxygen species (ROS). Additionally, interactions of the silanol groups on the SiNP surface and phospholipids in the cell membrane were responsible for the associated cell damage.
Overall, the results present the new AF4-ICP-MS technique as a powerful tool for quantitatively determining cytotoxicity induced by metal nanoparticles of varying sizes. The insights from this study also provide a solid groundwork for future studies looking into the evaluation of nanoparticle exposure risks and their potential burden on human bodies. “Our study aim was to come up with a facile analysis technique that would aid in the mission of minimizing potential health damage from nanoparticles. We are hopeful that the toxicological information provided by our study will help establish criteria for the proper utilization and regulation of nanoparticles in industries, the medical field, and even in daily use items containing nanoparticles,” concludes Dr. Tanaka.
